
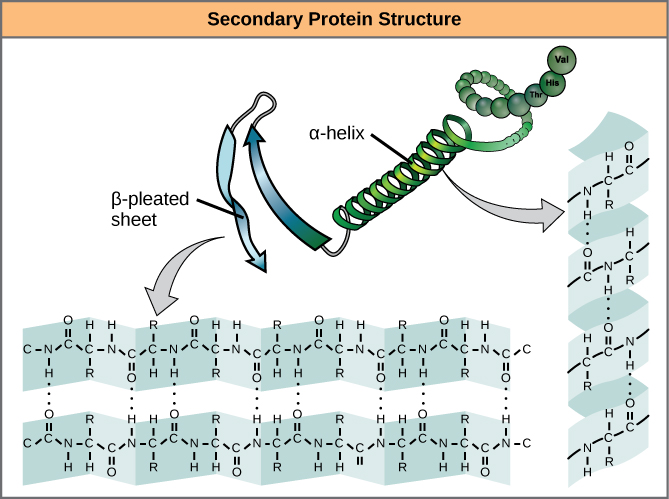
Now the next level of structure, this is just the order, this is how we form our polypeptide, but how does a polypeptide start getting bent into these shapes to be able to do the different things that it needs to do? Well the second, the second order of our structure, or I could say the secondary structure, secondary structure, this is due to interactions of the peptide backbone. It's coding for what order do we put the different amino acids in, so this is just the order, the order, order of, of the amino, of the amino acids. The DNA, the information in DNA, that's essentially what it's coding. When we talk about the translation step, when we go from mRNA and we go to a ribosome and the tRNA brings the amino acids and puts them, and starts linking them together, it's setting up the primary, it's setting up the primary structure. Primary structure, and this is really just the sequence of the amino acids. The first degree of the structure we can call the primary structure. How do they get their structure? Well, one way to think about it is, there's different layers of the structure, or there's different degrees of structure. So how do proteins like hemoglobin, there's many, many other types of proteins that do many, many other types of things. But you see, they don't just go into some random configuration, they come into a configuration that is really good for doing what hemoglobin does, and that is being a transporter for oxygen, being a transporter for oxygen within red blood cells. Two of them have 141 amino acids, two of them have 146 amino acids, for a total of 574, 574 amino acids.
#Alpha helix protein backbone series
We've already spent a lot of time talking about proteins, and how they do a huge variety of things in biological systems, anything from acting as hormones to antibodies to providing structures in cells, signaling mechanism, a whole series of things and their ability to do all of those things in living systems comes out, it's a by-product of their structure, so what we want to talk about in this video is protein, protein structure, and to just get a high-level appreciation for protein structure, this is a hemoglobin molecule right over here, and this hemoglobin molecule, it's made out of four polypeptide chains. Īnother set of techniques using mass-spectroscopy is often used, but I'm not going to try to explain that here! Note however, this can get very complicated, since there may be multiple copies of one or more identical proteins within a complex, some proteins may be of similar sizes, some proteins may have been broken down into smaller parts, you may have not completely purified your protein. The number of different bands corresponds to the number of different proteins. You then stain the gel with something that interacts with all proteins but not the gel. Because different proteins are usually different sizes they move at different rates through the gel. The mixture is then dragged through a gel by applying an electric field. This (usually) results in all the individual proteins being separated and stretched out into negatively charged rods.
If you have a purified protein complex a very standard technique involves denaturing the protein in an anionic detergent (SDS). And, note that there is a π bond between the C and O, which makes available additional electron density to O. For example, it matters what else the atoms involved might be bonded to.īut the difference in electronegativity of C and O is 0.89 which is sufficient to polarize a bond. There are many factors that go into determining how polarized a bond is.

There are a few other ways it can happen, but it is usually that way.Īs for what you mentioned about the electronegativity difference between O and C, please understand that electronegativity difference is a rule of thumb, not a strict law. Most of the time, the attraction will be between an H and N, O or F. This is not a true bond, just an electrostatic attraction. When, either in separate or the same molecule, you have a partially positively charged hydrogen come close to certain kinds of partially negatively charged groups, then you get an electrostatic attraction between the two groups. You should probably review hydrogen bonding in the chemistry section.


 0 kommentar(er)
0 kommentar(er)
